Sulfation is the formation or build-up of lead sulfate crystals on the surface and in the pores of the active material of the batteries’ lead plates. If the battery is left unattended once sulfation starts it will form larger crystals on the plates and the battery capacity will get smaller and smaller as the sulfation builds until it reaches a stage in which the battery may not work at all.
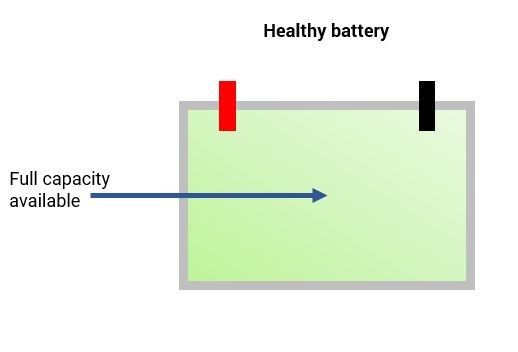
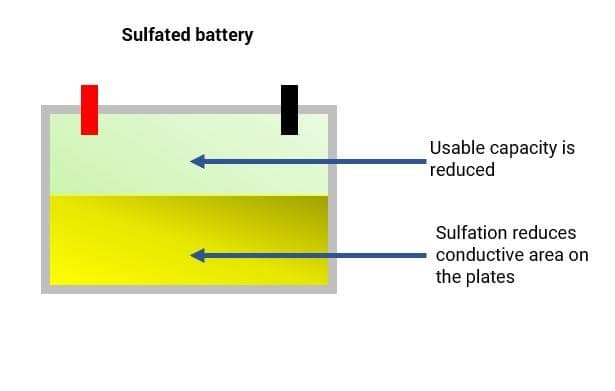
During normal use of the battery the formation of lead sulfate crystals is only temporary, they disperse during the recharging process. The issue with sulfation occurs when the larger lead sulfate crystals become permanent.
TYPES OF BATTERY SULFATION
There are two types of sulfation in lead acid batteries, reversible (also referred to as soft) and permanent (also referred to as hard). Fortunately their names are self-explanatory and imply the effects of sulfation to the battery. If sulfation is recognised early enough you can sometimes reverse the sulfation of the battery, this is something that should only be carried out by an experienced professional as can involve overcharging the battery and increasing the battery temperature. There has been some research into inverse charging for the recovery of sulphated lead acid batteries which can be found here if of interest.
An indication whether a lead acid battery sulfation can be reversed or not is visible on the voltage discharge curve. If a fully charged battery can hold a stable voltage profile on discharge there is a chance a reversal is possible, however if the voltage drops rapidly with load it is highly unlikely that it will be able to be reversed.
Permanent sulfation builds-up when a battery has been in a low state-of-charge for weeks or months. At this stage restoration of the battery, or reversal of the sulfation, is highly unlikely and as a result the battery will suffer the effects of permanent sulfation.
WHAT CAUSES A SULFATED BATTERY?
All lead acid batteries will suffer from sulfation during their lifetime as it is part of the chemical reaction of a battery. However, sulfation will build-up quicker when the battery is deprived of a full charge, under charged, stored at high temperatures, not used (charged and discharged) for prolonged periods of time or it if is stored without being fully charged first.
Battery sulfation is the most common cause of early battery failure in lead acid batteries.
Applications which can suffer from battery sulfation more frequently than others include starter batteries for cars and powersport vehicle. This can be due to short or infrequent journeys not giving the battery sufficient time to charge. Off-grid renewable applications such as wind and solar can also suffer from sulfation due to their intermittent nature not guaranteeing a full charge to the battery.
HOW TO FIND OUT IF A BATTERY IS SULFATED
If your battery is suffering from sulfation you will start to notice a decrease in the efficiency of the battery. The most common sign that a battery could be sulfated is when it does not hold a charge very well or doesn’t hold a charge at all, other signs include the battery going dead long before expected or electronic devices not getting the required power they need (ie dim headlights, weak AC, slow start-up). Visually from the outside you may not notice any difference in the battery appearance so the best way to find out if it is sulfation is to test the battery’s standing voltage with a multi-meter, if the voltage is less than 12.6 volts for an AGM battery or 12.4 volts for a starter battery it is a clear indication that the battery is undercharged which could be the result of sulfation.

THE EFFECTS OF A SULFATED BATTERY
Permanent battery sulfation can cause a variety of issues including:
- Loss of starting/cranking power
- Substantially longer charging times
- Increased heat build-up
- Shorter running times between charges
- dramatically shorter battery life
- complete battery failure
HOW TO PREVENT BATTERY SULFATION
The best way to prevent permanent battery sulfation is to maintain your lead acid battery, follow the recommended storage guidelines and follow lead acid battery charging best practices.
To prevent sulfation during storage a battery must be kept at a charge of at least 12.4 volts and be stored in an environment where temperatures do not exceed 75°F (24°C). For every 10°F increase above this temperature the rate of self-discharge of the battery doubles.
Even if you are storing a new, fully charged lead acid battery you still need to ensure the voltage does not drop below 12.4 volts. Applying a top up charge (also referred to as a maintenance charge) periodically will help prevent sulfation building up.

Sulfation is the number one reason you should not store your lead acid battery without charge. Once sulfation of the lead plates has happened, reversing the effects is highly unlikely, so it is essential to look after your batteries from the start.







